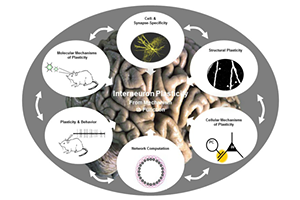
Individual Collaborations
In the course of the Research Unit 2143 about Interneuron plasticity, Prof.
Bartos and Prof. Diester investigate the role of parvalbumin and somatostatin positive interneurons for
cognitive behavior.
Csaba Földy is an Associate Professor and Co-Director of the Brain Research Institute.
Synapses enable communication between neurons in the brain. The Jonas group investigates how signals pass through these vital interfaces – a major undertaking in the field of neuroscience.
Understanding the function of the brain is a major challenge in the 21st century. The human brain comprises ~100 billion neurons, which communicate through ~10000 synapses per cell. Excitatory synapses use glutamate as a transmitter, whereas inhibitory synapses release Gamma-Aminobutyric acid (GABA). The group addresses two major questions. First, what are the biophysical signaling and plasticity mechanisms at glutamatergic and GABAergic synapses in the cortex? Second, how do specific synaptic properties generate higher network functions? In their work, the group combines nanophysiology, presynaptic patch-clamp and multi-cell recording, two-photon Ca2+ imaging, optogenetics, functional anatomy (“flash and freeze” electron microscopy), in vivo recording, and modeling. One focus is hippocampal mossy fiber synapses and output synapses of parvalbumin-expressing GABAergic interneurons
Experience-dependent changes in behavior are mediated by long-term functional modifications in brain circuits. We are interested in understanding the underlying mechanisms at the molecular, cellular and circuit levels. As a model system, we are using classical (Pavlovian) fear conditioning, a simple form of associative learning that is particularly suitable for study in rodents.
The inability to control or inhibit inappropriate fear responses is a hallmark of human anxiety disorders. We are investigating the cellular mechanisms underlying fear extinction, an associative learning process mediating inhibitory control of inappropriate fear behavior.
Using a multidisciplinary and integrated experimental approach in mice, we are combining in vitro and in vivo electrophysiology, imaging, molecular biology, genetics, and behavioral techniques to identify the synaptic and cellular constituents of neural circuits in the amygdala underlying the acquisition, encoding and extinction of fear memory – the microcircuitry of fear conditioning.
The Skinner Lab is part of “Krembil Computational Neuroscience (KCN)”
Focus: Neuronal and Neuronal Network Model Development, Use and Analyses
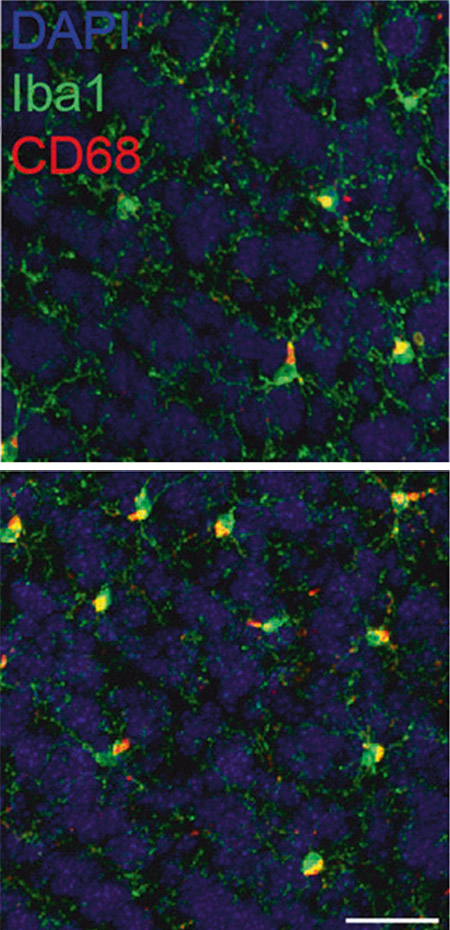
Neuroscience in Freiburg
Research Consortia