led by
P.D. Dr. Armin Just
We study the mechanisms governing blood flow and filtration rate in the kidney, ranging from local mediators and signaling pathways to the integrated function of pressure-dependent autoregulation.
Research
Our goal is to understand the mechanisms involved in the control of renal blood flow and glomerular filtration rate. This pertains to local mediators such as nitric oxide (NO), prostaglandins and EDHF, intracellular signaling pathways and connexins, hormones, renal nerves and the mediators and modulators of renal autoregulation.
Recent Studies
In a collaboration with C. Wagner and A. Kurtz from Regensburg, C. De Wit from Lübeck, and W.J. Arendshorst from Chapel Hill (Just et al 2009), we studied the role of the gap junction protein connexin 40 (Cx40) in renal blood flow autoregulation. Cx40 is known to be expressed in the relevant area of the kidney, (the so called juxtaglomerular apparatus, JGA), and to be involved in the regulation of renin. Our study revealed that Cx40 importantly contributes to the TGF-component of renal blood flow autoregulation. This function of Cx40 can only partially be replaced by another connexin, Cx45, when expressed under the Cx40-promotor instead of Cx40. Knockout of Cx40 alone, however, had no influence on the modulatory influence of NO or on vasomotor responses to norepinephrine, angiotensin II, or acetylcholine.
In Dautzenberg et al. 2011 we investigated the roles of the three isoforms of the NO-producing enzyme NO-synthase (NOS), i.e. endothelial (eNOS), neuronal (nNOS), and inducible NOS (iNOS) in modulating renal blood flow autoregulation. The isoforms are known to be expressed in different cell types: eNOS in the endothelial cells of the afferent arteriole, nNOS in the macula densa (i.e. the sensor of TGF), and iNOS in mesangial and vascular smooth muscle cells. nNOS- or iNOS-selective inhibitors in rats did not affect autoregulation, even though an inhibitor of all three NOS-isoforms induced the expected modulation in the same animals. nNOS-knockout (ko) mice showed the same autoregulation as their wild-type controls and responded normally to the non-selective NOS-inhibitor. However, eNOS-ko-animals failed to shift the balance of the autoregulatory mechanisms in response to NOS-inhibition, indicating an important role of eNOS for this modulatory influence of NO. Together, these results indicate, that the modulatory influence of NO on renal autoregulation is mediated predominantly if not exclusively via NO from eNOS, but not from nNOS or iNOS.
The dynamics of the mechanisms underlying endothelium-dependent vasodilation in the kidney were studied in Dautzenberg & Just 2013. Endothelium-dependent vasodilation is known to be mediated by nitric oxide (NO), prostaglandins (PG’s), and endothelium-derived hyperpolarizing factor(s) (EDHF). We studied the contribution and the time courses of these three components in the vasodilator responses to acetylcholine (ACh) and bradykinin (BK). We found that NO, PG, and EDHF contribute >50%, 20-40%, and <20%, respectively, and that EDHF acts considerably faster and more transiently (maximum after 16 s) than NO and PG (maximum after ~30 s). We also assessed the participation and dynamics of NO and PG in attenuating constrictor responses to norepinephrine and angiotensin II. We found that these attenuating influences are not constant over time, but exert their most prominent impact later than the peak of the constriction. Accordingly, these influences not only reduce the magnitude but also the duration of the constriction.
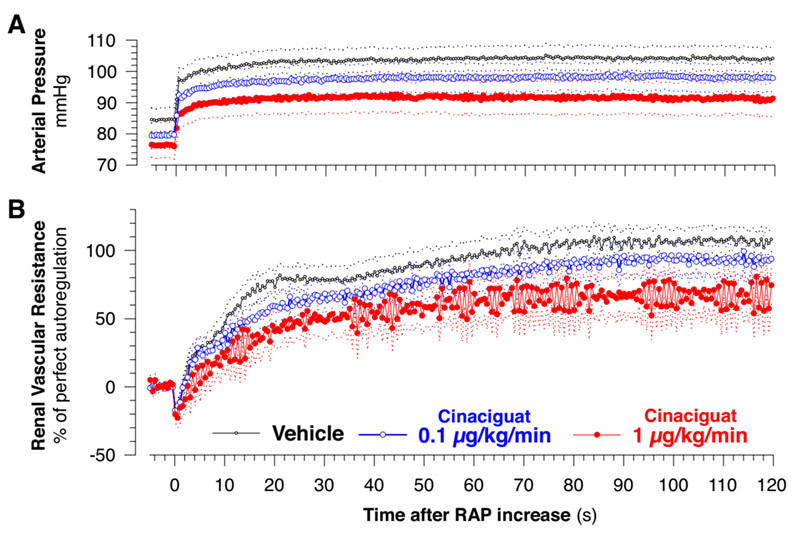
In (Dautzenberg et al. 2014) we studied the role of soluble guanylate cyclase (sGC) in the regulation of renal blood flow and glomerular filtration rate, and in the NO-dependent modulation of renal blood flow autoregulation. sGC is an enzyme producing cyclic guanosine monophosphate (cGMP), and is the main target for the effects of NO. Due to these features, sGC is a key player in the cGMP signaling cascade. Pharmacological agents have been developed targeting sGC directly, so called sGC-stimulators and -activators, some of which are already approved therapeutics. However., little is known about their influence on renal hemodynamics. Furthermore, although sGC is thought to be the main target for NO, other signaling pathways for NO have also been described. We therefore studied the impact of a prominent sGC-activator, Cinaciguat, on blood flow, filtration rate, and autoregulation, and also investigated the extent to which the effects of endogenous NO are mediated via sGC. Infusion of cinaciguat in anesthetized rats did not reduce blood flow or filtration rate, and only slightly impaired autoregulation, despite significant hypotension. This indicates safety of cinaciguat with regard to kidney function. Inhibition of endogenous NO production induced the known changes in blood pressure, renal blood flow, and autoregulation. Additional cinaciguate normalized these changes by ~80% for mean pressure and flow, and by ~90% for autoregulation, indicating that these effects of endogenous NO ar predominantly mediated via sGC.
Current work
Presently, we study role of endogenous prostaglandins and the influence of salt intake on renal blood flow autoregulation. In particular, we strive to find out whether vasodilator prostaglandins such as PGE2 and PGI2 might attenuate the contribution of MR in a similar way as observed previously for NO. This hypothesis is based on the known similarity of and interactions between the signaling pathways for PGE2 and PGI2 (via cAMP) and NO (via cGMP). We also inquire whether the constrictor prostaglandin TXA2 strengthens TGF and its autoregulatory contribution in vivo when interacting with MR and 3rd mechanism in the intact kidney in a similar way as has been described in the literature when TGF was studied in isolation. Finally, we intend to clarify, whether renal autoregulation is altered by salt intake, and the role of prostaglandins in such adaptation. The latter is based on literature reporting that TXA2 enhances TGF only during high, but not during low salt intake. To dissect the roles of the prostaglandin-producing enzymes cyclooxygenase COX1 and COX2 we studied RBF autoregulation before and afterr non-selective COX1/2- and after COX1- and COX-2-selective inhibitors, under normal, low salt and high salt diets.
Results so far generally indicate that non-selective COX1/2-inhibition, as well selective COX1- and COX2-inhibition do not affect MR, but depress the oscillation and strength of TGF, as well as total autoregulatory capacity. Salt restriction seems to slightly augment the oscillation of TGF, but MR, third mechanism, and total autoregulation were not affected by salt intake.
Techniques
We study anesthetized rats and mice including gene-knockout animals. We measure arterial pressure and renal blood flow using pressure transducers connected to arterial catheters and miniature ultrasound transit time flow sensors (Transonic®) implanted on the left renal artery. Glomerular filtration rate is measured by classical clearance using fluorescence-labeled sinistrin as marker with our customized analysis algorithm for improved accuracy. Experimental interventions include manipulation of arterial pressure and infusion of agents intravenously or directly into the renal artery. For analysis we employ computer-based analysis tools in the time and frequency domain, such as signal averaging, adapted normalization procedures, fourier transforms, and transfer function analysis.
Collaborations
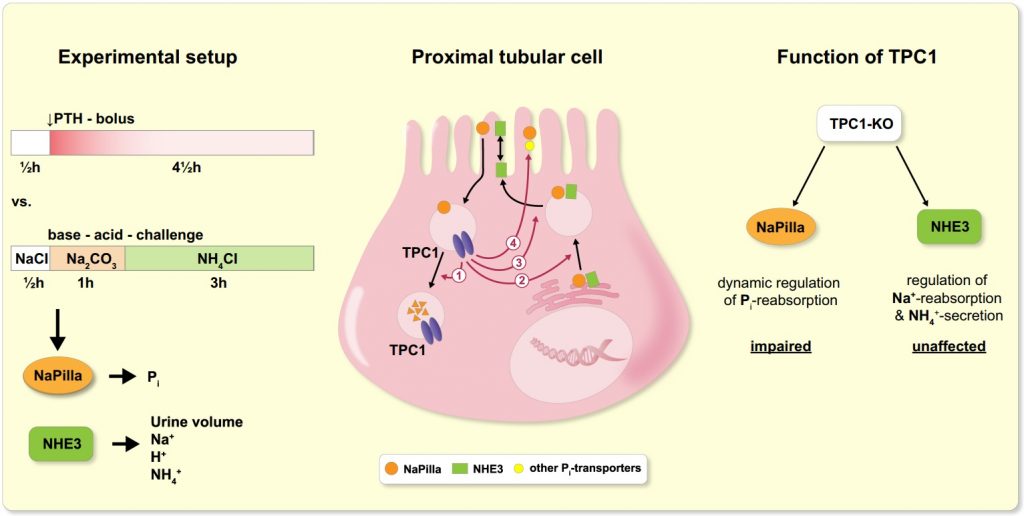
We recently studied the localization of TPC protein 1 (TPC1) in the kidney and its functional role in the dynamic regulation of tubular transport.
Two-pore channels (TPCs) constitute a small family of cation channels expressed in endo-lysosomal compartments, and have been characterized as critical elements controlling Ca2+-mediated vesicular membrane fusion, and thereby regulating endo-lysosomal vesicle trafficking. Exo- and endocytotic trafficking and lysosomal degradation are major mechanisms of adaption of epithelial transport – a prime example of highly regulated epithelial transport is the tubular system of the kidney.
We found that TPC1 is expressed subapically in the proximal but not distal tubule and plays an important role in the dynamic adaptation of proximal tubular phosphate reabsorption towards enhanced, but not reduced absorption.
Publications
Original Publications
Review
Editorials
Complete Publication List