led by
Prof. Dr. Marlene Bartos
We are interested in understanding how dendritic integration of activity in principal cells and GABAergic interneurons influences microcircuit function and behavior.
Research
A fundamental and fascinating feature of the mammalian brain is its capacity to acquire and store novel information. Our research is focused on how memory is represented in neuronal networks. We aim to understand the mechanisms underlying the emergence of learning-associated active cell populations (cell assemblies) that represent new memories. We focus on the rodent dentate gyrus (DG), the input region of the hippocampus known to be functionally vital for acquiring new memories in many species, including humans and rodents. Our work has clarified the cellular and synaptic properties of neurons and synapses in the DG circuitry, and mechanisms underlying the synchronization of neuronal networks for the encoding of information. We have investigated the cellular, synaptic and network mechanisms important for the development of neuronal networks, with a focus on GABAergic inhibitory cells.
Our major research topics are:
- to understand the spatial and temporal emergence of learning-associated cell assemblies representing new memories.
- to delineate the nature and relevance of the major functional (cellular, synaptic, plasticity) and structural changes underlying cell assembly formation.
- to understand the functional and dynamic characteristics of synaptic communication among cells and their role in information processing in cortical microcircuits.
- to identify the role of the highly varied GABAergic cell population in neuronal network function and cell assembly formation.
- to examine the dysfunction of cellular components in specific mouse models underlying neuronal diseases.
Techniques
Miniscope Imaging
We use Inscopix nVoke systems to image Ca2+ signals at one photon cellular resolution combined with optogenetic stimulation in the same field of view to investigate hippocampal and prefrontal cortex cell activity and circuitry in freely behaving animals.
We aim to understand the longitudinal involvement of cell ensembles in prefrontal cortex and hippocampal subregions during working memory, social memory and spatial coding/discrimination. We want to understand the circuit dynamics of learning, memory consolidation and recall of recent and remote memory, and thus unravel the contribution of different cell types and neuromodulators to the memory representation in the brain.
2-Photon population imaging
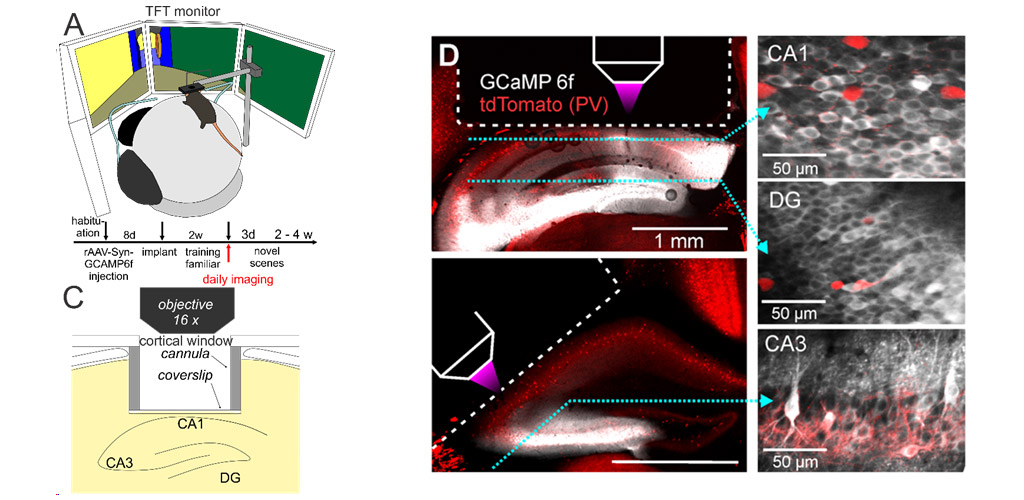
We study the emergence and activity of hippocampal memory engrams in awake, behaving animals, using two-photon calcium imaging in the dentate gyrus and hippocampal subfields CA1-3 of head-fixed mice. Mice perform contextual discrimination tasks in a virtual environment displayed on screens around them. We investigate how principal neurons in different hippocampal subfields represent spatial contexts, how these representations develop with increasing familiarity, how local microcircuit mechanisms underly the formation of neuronal ensembles, and how the activity of local inhibitory circuits is modulated by sensory experience and novelty detection. Our goal is to decipher how neuronal circuits in the hippocampus store and retrieve the contents of complex episodic memories.
Connectivity of Neuronal Microcircuits
For the function of a given neuron (type) to be accurately assessed, it is essential to map the pre- and post-synaptic partners of that cell (type). We use retrograde markers, such as fluorescent-dye-conjugated Cholera Toxin Beta subunit (CTB), as well as retrograde & anterograde Adeno-Associated Viruses (AAV) of various serotypes, and Canine Adeno-Virus type 2 (CAV2) to fluorescently label neuronal populations. We obtain further specificity by using selective viral promoters, or Cre-recombinase-expressing transgenic mice, to target specific neuronal sub-populations based on their neurochemical identity or post-synaptic target area. Further, these viruses can also be used to simultaneously express genetically-encoded light-controlled ion channels (opsins) to activate or inhibit these different cell types, either in vivo or in vitro, enabling analysis of both structural and functional connectivity. We also investigate the presynaptic inputs to different cell types using monosynaptic rabies virus tracing, allowing us to assess neuronal sub-type-dependent connectivity motifs between identified pre- and post-synaptic neurons in the brain.
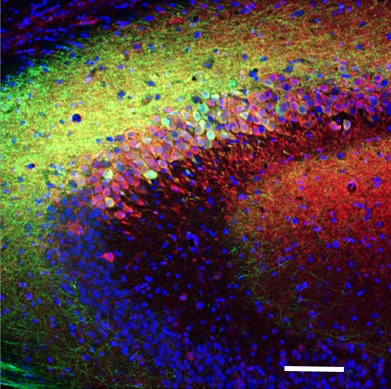
Left image shows the Hippocampus of a transgenic mouse that expressed cre under the Somatostatin promoter after injection of a cre-dependent, GFP-expressing virus into the Dentate Gyrus (green). Post-hoc immunolabelling for Calbindin (Ca2+ binding protein; red) and mGluR1α (metabotropic Glutamate receptor; blue) was also performed. An enlarged view is shown on the right.
Single unit silicon probe recording
We probe neural network dynamics using large-scale single-unit recording from head-fixed mice navigating virtual environments (A). With multi-shank silicon probes and optical fiber implants we investigate the activity of hippocampal principal cells and optogenetically identified interneurons (B). Using linear silicon probes we furthermore probe the activity of neurons across multiple prefrontal cortex areas in awake mice (C). Our goal is to understand how neural ensembles in hippocampus and cortex dynamically encode behavioural variables.
Interneuron diversity
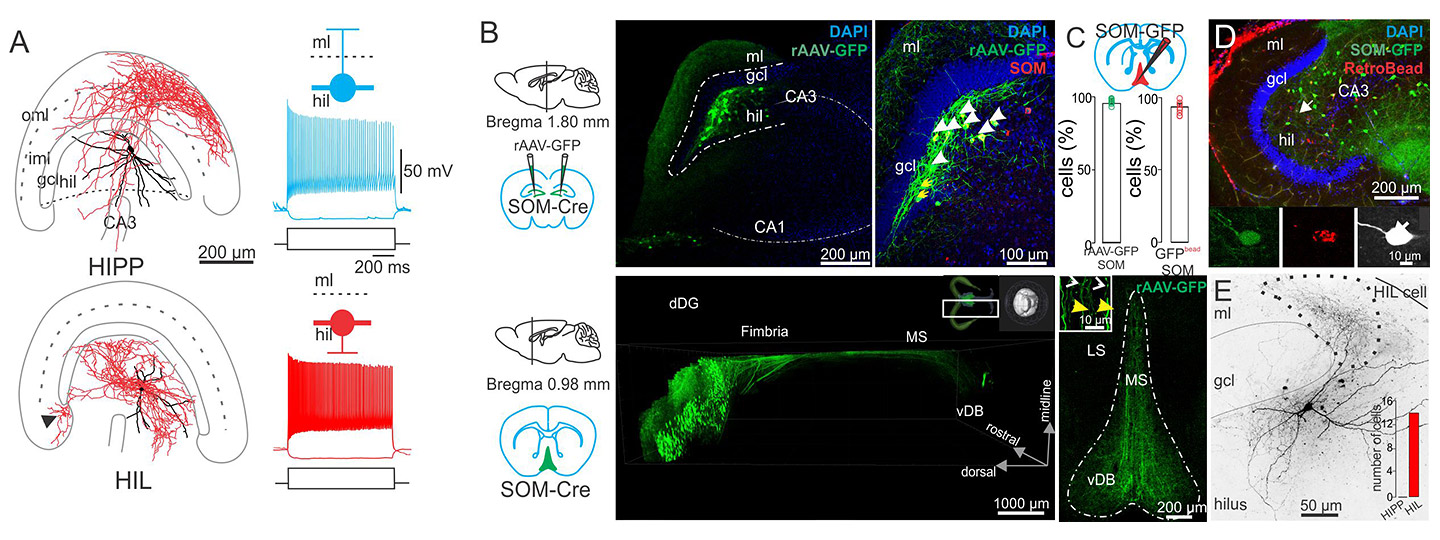
During the course of the last ~25 years, there was an explosion of studies on the neurochemical, morphological, physiological and pharmacological properties of interneurons, which gave rise to the identity of a multitude of anatomically and functionally distinct GABAergic interneuron types. These studies revealed that the cell body, axon-initial segment and dendritic domains of pyramidal cells and interneurons are targeted by distinct interneuron types (Markram et al., 2004; Bartos et al., 2014), indicating that compartment-specific computational operations on the level of individual cells are controlled by specialized inhibitory cells. Interneuron diversity was largely investigated in the hippocampal CA1-3 and neocortical areas. We focus our work on the dentate gyrus, the input gate of the hippocampus using intracellular labelling of cells in slice preparations with subsequent antibody labelling, confocal and electron microscopy. Moreover, by using transgenic mice with viral expression we label local and long-range projecting interneuron types.
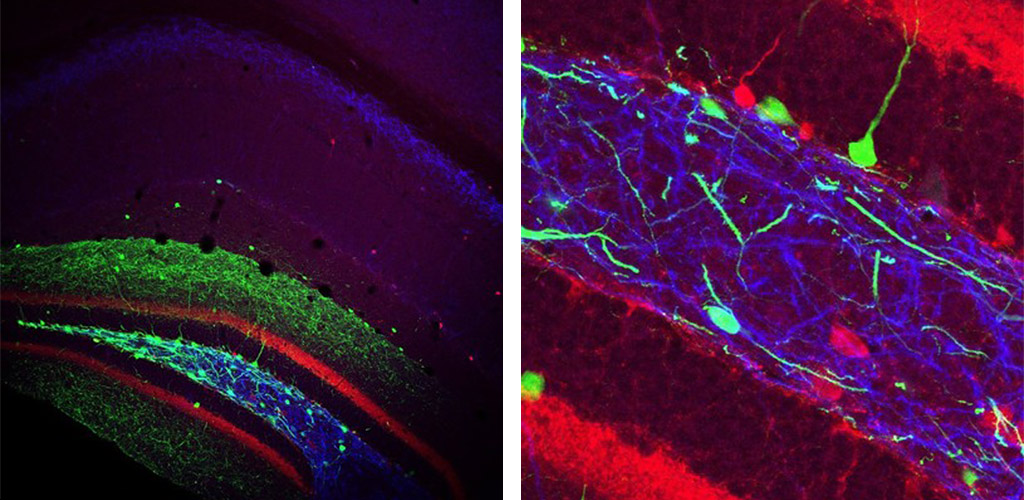
A) Morphological reconstructions and electrophysiological responses to current injection for two different kinds of Somatostatin-expressing interneurons with cell bodies in the Hilus. B) Injecting cre-dependent virus into SOM-cre mice labels Hilar neurons with GFP (green), and axons can be traced to target areas, such as the Medial Septum (lower panel). DAPI (blue) is a cellular counterstain. Immunolabelling for Somatostatin was used to confirm cell type (upper right panel). C) Retrobeads injected in the Medial Septum labelled (red) confirmed that the GFP-expressing axons originated from the Hilar cells. D) Example images of intracellularly filled, retrobead-labelled Hilar, Somatostatin-expressing interneurons.
Plasticity
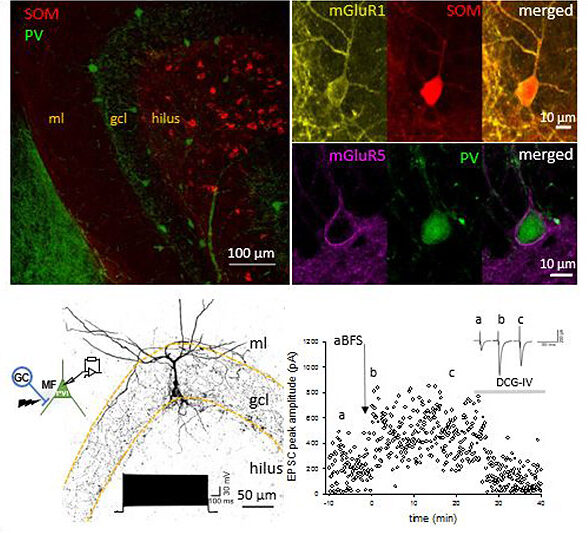
GABAergic inhibitory interneurons (INs) in the mammalian cortex are characterized by a broad diversity based on their molecular, morphological and physiological characteristics. The various IN types are thought to control the activity and excitability of their target cells in a compartment-specific manner. Recent investigations in our group indicate that some IN types are under plasticity control. In our research we focus on the mechanisms that allow long term strengthening or weakening of glutamatergic synaptic inputs onto parvalbumine (PV) and somatostatin (SOM)-expressing interneurons (PVIs, SOMIs, respectively). We aim to understand how these synaptic plastic changes contribute to the overall state of the neural network activity in which these INs are embedded in, information processing and the behavior output. To accomplish these aims, we use a multidisciplinary approach combining in vitro whole-cell recordings, shRNA for interference with synaptic plasticity induction, in vivo single unit recordings of optotagged IN types and behavioral analysis. We focus these investigations on PVIs and SOMIs of the dentate gyrus in mice. We previously showed that mossy fiber synapses originating from dentate gyrus granule cells mediate long-term potentiation (LTP) onto both IN types. This form of LTP depends on the activation of group I metabotropic glutamate receptors (Sambandan et al., J Neurosci 2010; Hainmueller & Bartos, PNAS USA 2014; Yuan et al., eLife 2017).
Upper Left: Immunolabelling in the Dentate Gyrus for Parvalbumin (PV; green) and Somatostatin (SOM; red). Upper Right: the metabotropic Glutamate receptor type 1 (mGluR1; yellow) is expressed by SOM interneurons (red, top right), and the metabotropic Glutamate receptor type 5 (mGluR5; magenta) is expressed by PV interneurons (green). Lower Left: morphology and electrophysiology of a paired recording from a Dentate Gyrus Granule Cell to a PV-expressing interneuron. Lower Right: associative Burst Frequency Stimulation produces a long-lasting enhancement of the interneuron excitation that could be reversed by DCG-IV.
2-Photon population imaging videos
Selected Publications
2024
- Huang L-W, Torelli F, Chen H-L, Bartos M (2024) Context and space coding in mossy cell population activity. Cell Reports 43(7):114386. doi: 10.1016/j.celrep.2024.114386. Online ahead of print.
- Muysers H, Chen H-L, Hahn J, Folschweiller S, Sigurdsson T, Sauer JF, Bartos M (2024) A persistent prefrontal reference frame across time and task rules. Nature Communications DOI 10.1038/s41467-024-46350-4
- Kaufhold D, Maristany de las Casas D, Ocana-Fernandez M A, Cazala A, Yuan M, Kulik A, Cholvin T, Steup S, Sauer JF, Eyre MD, Elgueta C, Struber M, Bartos M (2024) Spine plasticity of dentate gyrus parvalbumin-positive interneurons is regulated by experience. Cell Reports 43: 113806 https://doi.org/10.1016/j.celrep.2024.113806
- Sylte OC, Muysers H, Chen HL, Bartos M, Sauer JF (2024) Neuronal tuning to threat exposure remains stable in the mouse prefrontal cortex over multiple days. PLOS Biology22(1):e3002475. doi: 10.1371/journal.pbio.3002475. eCollection 2024 Jan.
2023
- (2023) Synaptic plasticity at the dentate gyrus granule cell to somatostatin-expressing interneuron synapses supports object location memory. Proc Natl Acad Sci U S A 120:e2312752120. doi: 10.1073/pnas.2312752120. Epub 2023 Dec 13.
- Hanganu-Opatz IL, Klausberger T, Sigurdsson T, Nieder A, Jacob SN, Bartos M, Sauer JF, Durstewitz D, Leibold C, Diester I (2023) Resolving the prefrontal mechanisms of adaptive cognitive behaviors: A cross-species perspective. Neuron 111:1020-1036.
2022
- Cholvin T, Bartos M (2022) Hemisphere-specific spatial representation by hippocampal granule cells. Nature Com. DOI: 10.1038/s41467-022-34039-5.
- Strüber M, Sauer JF, Bartos M (2022) Parvalbumin expressing interneurons control spike-phase coupling of hippocampal cells to theta oscillations. Sci Rep. 112:1362
- Sauer JF, Folschweiller S, Bartos M (2022) Topographically organized representation of space and context in the medial prefrontal cortex. PNAS 119 (6) e2117300119.
2021
2020
- Hainmueller T, Bartos M (2020) Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nature Rev Neurosci, 21:153-68.
- Paschen E, Elgueta C, Heining K, Vieira D, Kleis P, Orcinha C, Häussler U, Bartos M, Egert U, Janz P, Haas C (2020) Hippocampal low-frequency stimulation prevents seizure generation in a mouse model of mesial temporal lobe epilepsy. elife 10.7554/elife.54518.
2019
- Elgueta C, Bartos M (2019) Dendritic inhibition differentially regulates excitability of dentate gyrus parvalbumin-expressing interneurons and granule cells. Nature Comm 10:5561. https://doi.org/10.1038/s41467-01913533-3.
- Eyre MD, Bartos M (2019) Somatostatin-Expressing Interneurons Form Axonal Projections to the Contralateral Hippocampus. Front Neural Circuits, 13:56. Published 2019 Aug 23. doi:10.3389/fncir.2019.00056
- Holz A, Mülsch F, Schwarz MK, Hollmann M, Döbrössy MD, Coenen VA, Bartos M, Normann C, Biber K, van Calker D, Serchov T (2019) Enhanced mGlu5 signaling in excitatory neurons promotes rapid antidepressant effects via AMPA receptor activation. Neuron. 2019 Jul 23. pii: S0896-6273(19)30637-3. doi: 10.1016/j.neuron.2019.07.011.
2018
- Hainmüller T, Bartos M (2018) Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558:292-96.
- Sauer JF, Strüber M, Bartos M (2018) Recording Spatially Restricted Oscillations in the Hippocampus of Behaving Mice. Jove-j Vis Exp doi:10.3791/57714.
2017
- Strüber M, Sauer JF, Jonas P, Bartos M (2017) Distance-dependent inhibition supports focality of gamma oscillations. (2017) Nature Commun. DOI: 10.1038/s41467-017-00936-3.
- Yuan M, Meyer T, Benkowitz C, Savanthrapadian S, Ansel-Bollepalli L, Foggetti A, Wulff P, Alcami P, Elgueta C, Bartos M (2017) Somatostatin-positive interneurons in the dentate gyrus of mice provide local- and long-range septal synaptic inhibition. eLife 2017;6 e21105
- Biskamp J, Bartos M, Sauer JF (2017) Organization of prefrontal network activity by respiration-related oscillations. Sci Rep 7:45508
2016
2015
- Elgueta C, Köhler J, Bartos M (2015) Persistent discharges in dentate gyrus perisoma-inhibiting interneurons require hyperpolarization-activated cyclic nucleotide-gated channel activation. J Neurosci 35:4131-4139
- Sauer JF, Strüber M, Bartos M (2015) Impaired fast-spiking interneuron function in a genetic mouse model of depression. eLife 2015;10.7554/eLife.04979
- Strüber M, Jonas P, Bartos M (2015) Strength and duration of perisomatic GABAergic inhibition depend on distance between synaptically connected cells. PNAS USA ePub ahead of print; doi:10.1073/pnas.1423628112
2014
- Hainmüller T, Krieglstein K, Kulik A, Bartos M (2014) Joint CP-AMPA and group I mGlu receptor activation is required for synaptic plasticity in dentate gyrus fast-spiking interneurons. PNAS USA 111:13211-13216.
- Savanthrapadian S, Meyer T, Elgueta C, Booker S, Vida I, Bartos M (2014) Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks. J Neurosci 34:8197-8209.
2013
2012
- Bartos M, Elgueta C. (2012) Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol (Lond) 590:669-681.
- Sauer J-F, Strüber M, Bartos M (2012) Interneurons provide circuit-specific depolarization and hyperpolarization. J Neurosci 32:4224-4229.
2011
2010
2009
2008
2007
2006